
There is a growing consensus in the automotive industry that the future of land transportation lies in electric propulsion. While e-mobility is not a new concept—historically, internal combustion engines (ICE) and electric cars coexisted—there is an ongoing debate about the optimal method for supplying power to electric motors. Currently, most vehicles utilize battery packs, but hydrogen fuel cells present an alternative.
 Honda Clarity Fuel Cell Vehicle
Honda Clarity Fuel Cell VehicleWhat is a Fuel Cell?
Concerned about the limited energy capacity of current battery technologies, some experts have proposed a different solution: generating electricity onboard the vehicle without the need for heavy batteries. The technology is not new; NASA first utilized fuel cells in the 1960s for the Gemini and Apollo missions. In the automotive sector, proton exchange membrane fuel cells (PEMFC) are predominantly employed.
So, how is electricity generated in a fuel cell? The process is relatively simple, involving three main components: the anode, the cathode, and the separator. At the anode, hydrogen (H2) is supplied from a tank. Hydrogen consists of two electrons and two protons. The positively charged protons pass through the separator, while negatively charged electrons flow through an external circuit, producing electric current.
At the cathode, atmospheric oxygen combines with the protons that have passed through the separator and the electrons from the external circuit. This reaction creates water, which is expelled from the vehicle's exhaust—a tailpipe from which one could technically drink!
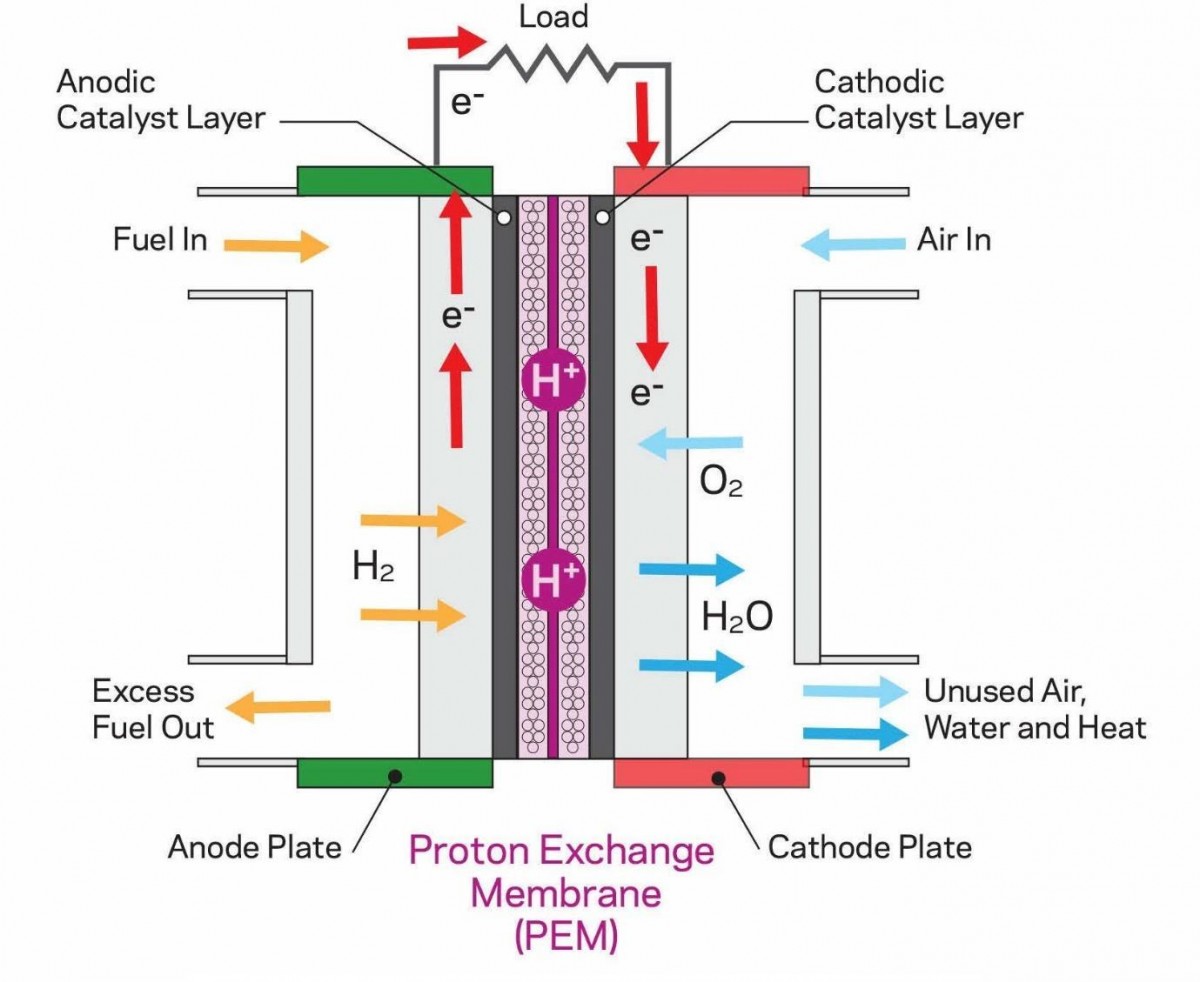 Nafion Proton Exchange Membrane Fuel Cell
Nafion Proton Exchange Membrane Fuel CellHydrogen Production
While hydrogen is the most abundant element in the universe, it is not found in its free form on Earth, necessitating methods for its extraction. Currently, there are four primary methods to produce hydrogen.
Gray hydrogen is produced via steam reforming of natural gas, where high heat and pressure convert natural gas into hydrogen and carbon dioxide (CO2). This method is the least environmentally friendly.
Blue hydrogen shares similarities with gray hydrogen but employs carbon capture and storage technologies during extraction, preventing CO2 emissions into the atmosphere.
Green hydrogen is derived from electrolysis, the reverse process of a fuel cell, which uses electric current to split water into hydrogen and oxygen.
Additionally, there is turquoise hydrogen, produced by passing natural gas through molten metal, yielding hydrogen and carbon. Though this technology shows promise, it is not yet ready for widespread implementation.
Among current methods, green hydrogen holds the most potential for sustainable and environmentally friendly production, provided the necessary electricity is sourced from renewable energy such as wind or solar.
 Hydrogen Production Through Sustainable Electrolysis
Hydrogen Production Through Sustainable ElectrolysisHydrogen States
Hydrogen can exist in gas or liquid states, each with its own advantages and disadvantages.
Because hydrogen is the least dense element, it must be compressed significantly for vehicular use. In current applications, hydrogen is compressed to 700 bars, necessitating specialized and costly tanks.
An alternative is to store hydrogen in liquid form, which requires cooling to -253°C (20°C above absolute zero). Achieving either state of hydrogen requires substantial energy—whether for high-pressure compression or extreme cooling.
However, the energy density of hydrogen is remarkable; 1 kg contains 33.3 kWh, offering 167 times more energy per kilogram than leading battery packs!
 Toyota Mirai’s Gaseous H2 Tanks are Made from Polyamide and Carbon Fiber
Toyota Mirai’s Gaseous H2 Tanks are Made from Polyamide and Carbon FiberBatteries vs. Hydrogen
So, which method is preferred: transporting electricity or generating it onboard? Currently, the automotive industry leans towards battery packs for several reasons.
The efficiency of PEMFCs hovers around 60%. In contrast, the efficiencies of steam reforming and electrolysis are approximately 70% and 75%, respectively. Hydrogen compression achieves about 89% efficiency while liquefaction has an efficiency of about 66%. Below is a summary table that compares these efficiencies between battery packs and hydrogen, using the most efficient scenarios for hydrogen.
| Efficiency Comparison | Hydrogen | Battery Pack |
|---|---|---|
| Electrolysis (%) | 75 | - |
| Hydrogen Compression (%) | 89 | - |
| Fuel/Grid Transport (%) | 80 | 93 |
| Fuel Cell (%) | 60 | 94 |
| Permanent Magnet Motor (%) | 94 | 94 |
| DC/AC Conversion (%) | 93 | 93 |
| Charging (%) | - | 94 |
| Transmission (%) | 95 | 95 |
| Total Efficiency (%) | 27 | 73 |
The efficiency data suggests that hydrogen fuel cell technology offers performance comparable to traditional petrol engine ICE vehicles. However, given the current state of technology, fuel cells are not yet practical for passenger vehicles, which is why most manufacturers are favoring battery packs despite their limitations.
It is also important to note that hydrogen refueling costs 7-8 times more than charging an electric car at home for the same distance. Nonetheless, hydrogen could play a role in heavy trucking due to its high energy density, provided we can develop sustainable production methods.





